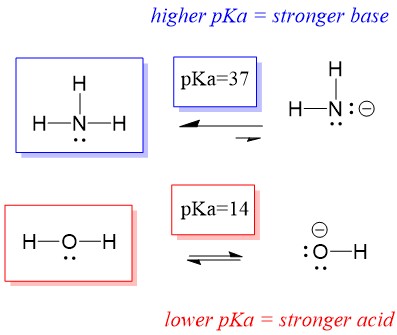
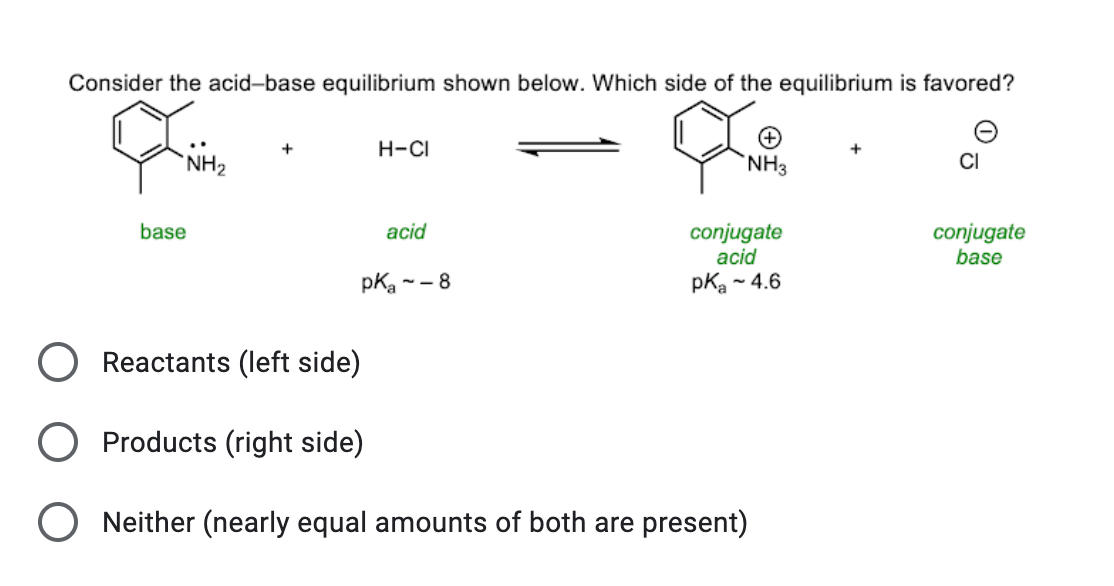
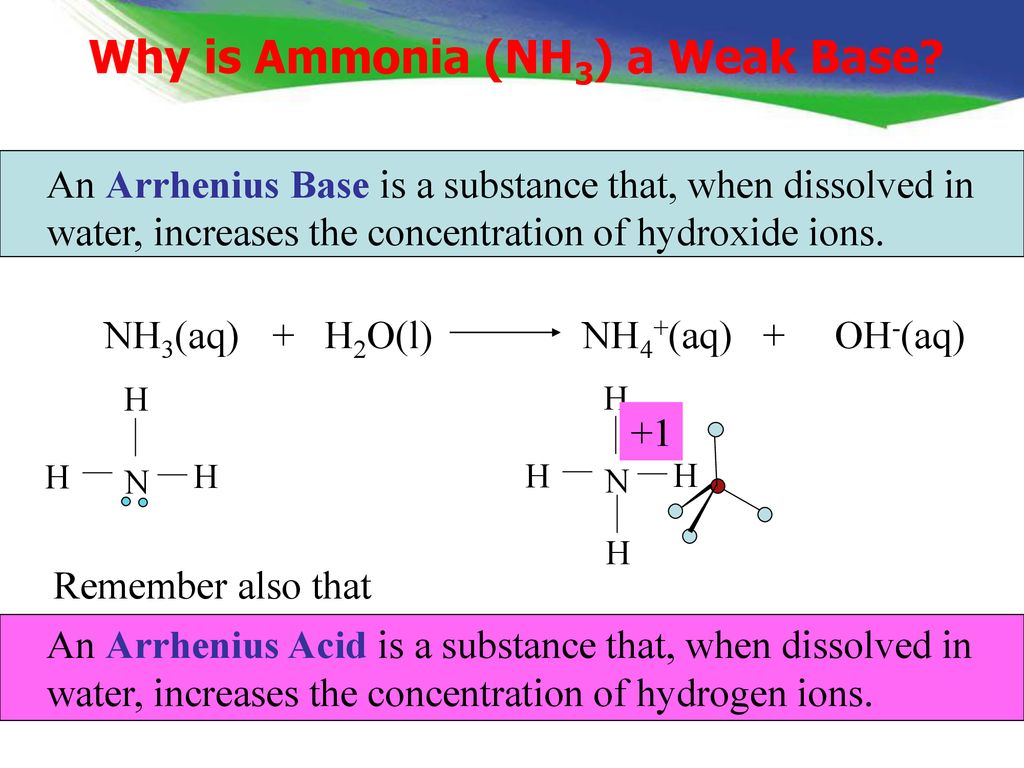
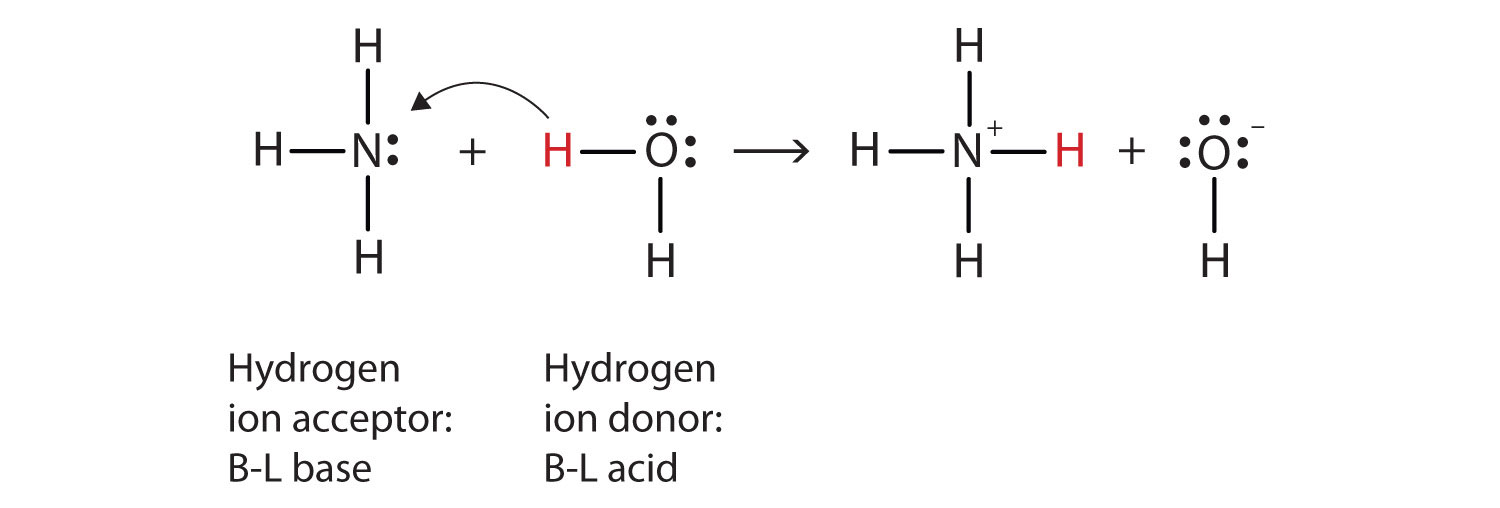
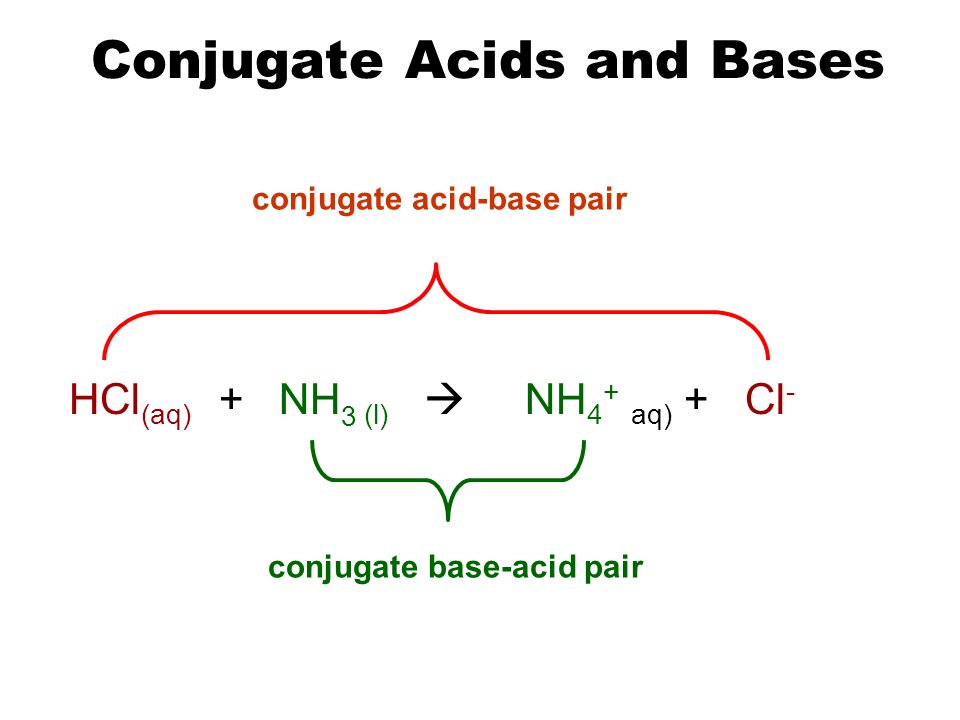
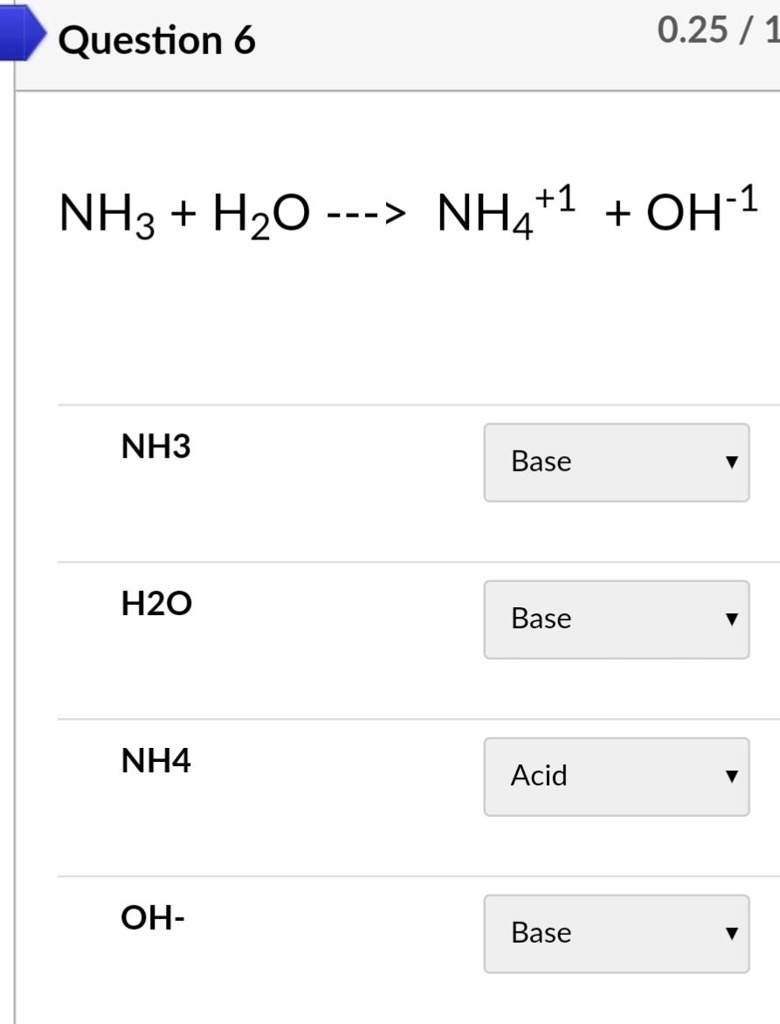
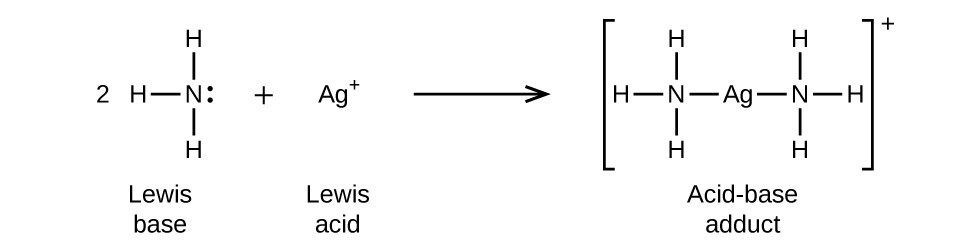
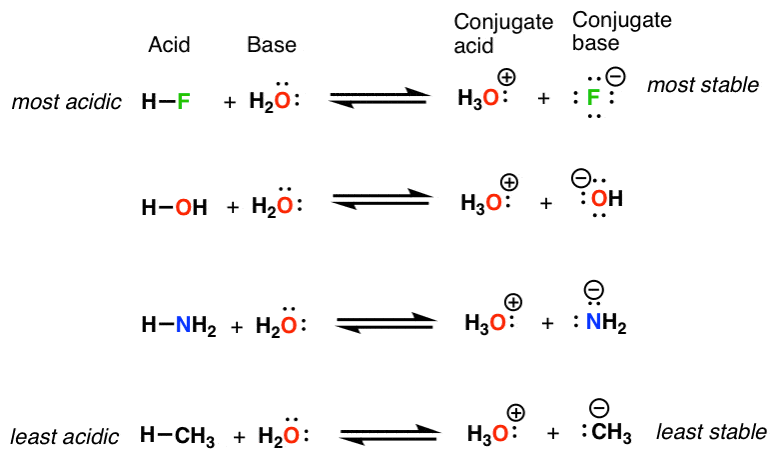
NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in NH4Cl? | Study.com

organic chemistry - Why In This Reaction Acetic Acid is strong acid and NH3 is strong base ?please explain in details and thanks for answer - Chemistry Stack Exchange